Retinitis Pigmentosa : Fasting , Autophagy , Stem Cells and more
Explore the latest updates on retinitis pigmentosa, including the role of fasting, autophagy, and stem cells in treatment. Learn about the symptoms, causes, complications, and the hereditary nature...
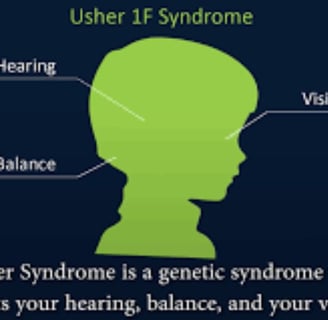
NERVOUS SYSTEM
Dr Hassan Alwarraqi
9/21/202410 منٹ پڑھیں
Retinitis Pigmentosa : Fasting , Autophagy , Stem Cells and more
Retinitis pigmentosa (RP) refers to a series of rare eye disorders that influence the retina,
the light-sensitive layer situated at the back of the eye.
The disease causes a progressive degeneration of retinal cells, leading to a decline in vision.
RP is a genetic condition that is present from birth
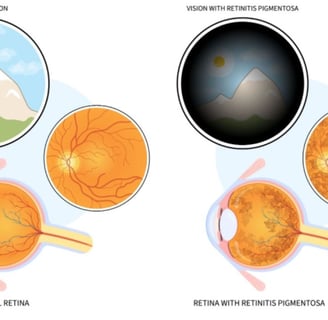
Retinitis pigmentosa is a hereditary eye condition that leads to vision impairment.
(RP) induces a slow breakdown of retinal cells, which contributes to a gradual loss of vision over time
retinitis pigmentosa causes
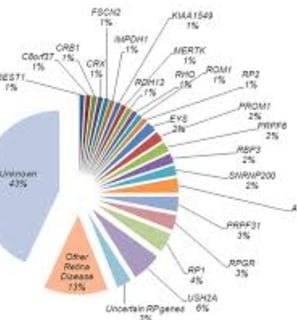
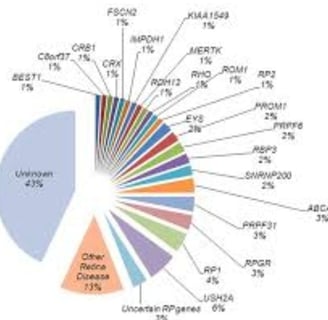
Each patient may carry a variant in one or more of approximately 100 genes.
Such variants ultimately interfere with the functioning of retinal cells, thereby affecting the retina's ability to perceive light.
Certain drugs are known to induce retinopathy with a notable preference for the retinal pigmented epithelium.
This includes antimalarial like quinine, hydroxychloroquine, and mefloquine, as well as phenothiazines, indomethacin, ethambutol, and desferrioxamine
retinitis pigmentosa symptoms
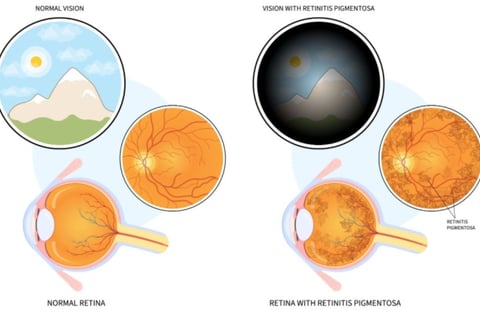
Impaired vision under poor lighting conditions or in darkness, including shadowy environments.
Limited ability to detect objects within the peripheral visual field.
A gradual reduction in the total extent of clear vision.
Frequent occurrences of stumbling or tripping over peripheral objects.a person with retinitis pigmentosa see
In advanced stages, central vision may also be impacted, leading to blurriness and areas of missing vision
Initial Symptoms: Loss of ability to see at night and a reduction in peripheral vision.
Progressive Symptoms: Severe vision loss potentially resulting in blindness.
Diagnosis Process: Conducting a dilated eye examination, performing electroretinography (a retinal diagnostic test), and carrying out genetic testing.
which can ultimately result in a phenomenon known as "tunnel vision." While complete blindness is rare, the onset of symptoms typically occurs gradually, often beginning in childhood.
linked to genetic mutations in nearly 100 different genes.
the progressive degeneration of rod photoreceptor cells located in the retina. which safeguards cone cells from apoptosis.
retinitis pigmentosa diagnosis
Eye sight evaluation
Examination using an ophthalmoscope
Refraction testing
Retinal assessment
Electroretinography testing
Visual field analysis
Genetic assessment by a licensed genetic counselor
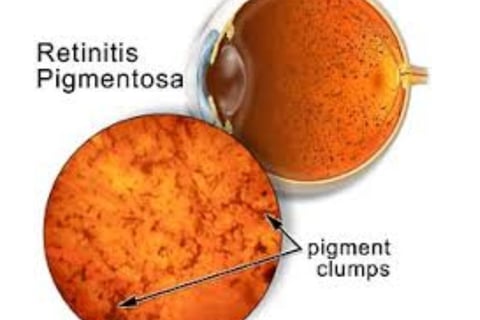
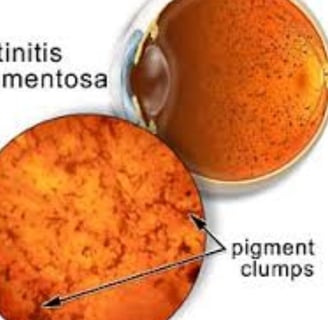
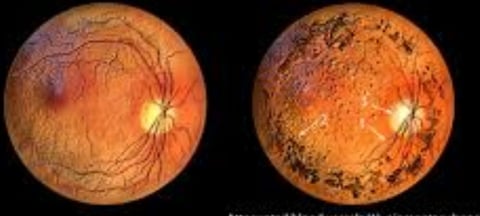
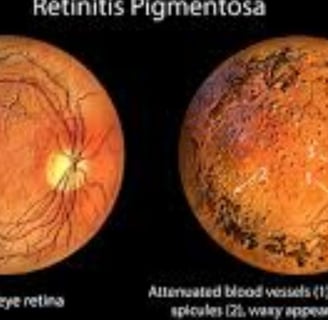
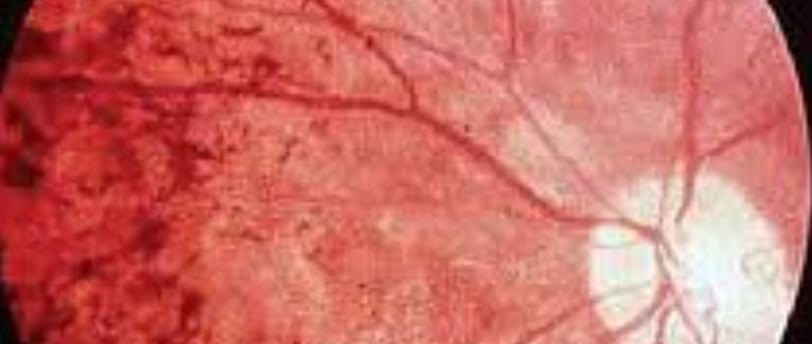
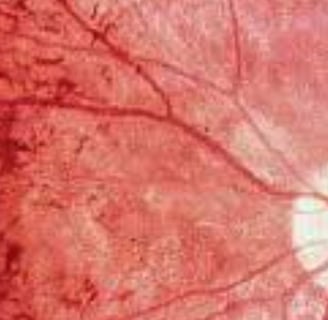
Diagnosis is achieved through a comprehensive eye examination, which may reveal dark pigment deposits resulting from the breakdown of retinal pigmented epithelial cells that contain melanin.
retinitis pigmentosa treatment
There is no known cure for retinitis pigmentosa.
you can't prevent RP
low vision aids, portable lighting solutions, and training in orientation and mobility.
Vitamin A palmitate supplements may help slow the progression of the disease.
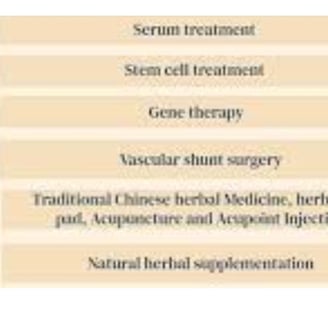
In certain cases of severe retinitis pigmentosa, a visual prosthesis may be considered as a treatment option.
gene therapy available for patients with Leber congenital amaurosis type 2, 50% of the patients who undergo the treatment
the etiology is quite variable
Treatment Options: Implementation of low vision aids and vision rehabilitation programs
retinitis pigmentosa complications
is characterized by a progressive decline in visual acuity, which may manifest either slowly or more rapidly,
but usually unfolds over an extended period. Those suffering from retinitis pigmentosa may additionally face the onset of cataracts at an early age and may experience retinal swelling, clinically known as macular edema
retinitis pigmentosa inheritance
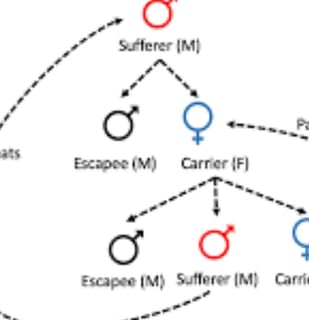
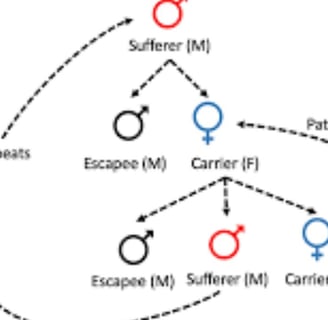
Retinitis pigmentosa often has an autosomal dominant inheritance pattern ,
which means one copy of an altered gene in each cell is sufficient to cause the disorder
Most people with autosomal dominant retinitis pigmentosa have an affected parent and other family members with the disorder
requiring that the gene be inherited from both parents for the disease to develop
Nonetheless, there are cases where dominant genes and genes on the X chromosome are associated with the condition, allowing for the possibility of the disease being transmitted from only one parent
retinitis pigmentosa treatment update
Theories That Support That Autophagy Is Decreased in RP
Autophagy Dysfunction and Oxidative Stress, Two Related Mechanisms Implicated in Retinitis Pigmentosa
retinitis pigmentosa age of blindness
Typically, this disorder is diagnosed in children, adolescents, and young adults.
It is recognized as a progressive condition, with variations in the rate of progression and the level of visual loss experienced by different individuals.
Many individuals with retinitis pigmentosa (RP) may become legally blind by the age of 40,
often having a central visual field measuring less than 20 degrees in diameter.
fasting Autophagy stem cells and Oxidative Stress, in Retinitis Pigmentosa
fasting, autophagy, oxidative stress, and stem cells play crucial roles in this condition.
Fasting and Autophagy
Fasting: Intervals of refraining from food intake can stimulate autophagy,
a crucial cellular process that facilitates the degradation and recycling of damaged cellular components.
Autophagy in Retinitis Pigmentosa (RP): Autophagy is associated with the elimination of impaired photoreceptor cells and aids in the preservation of the surviving cells.
Oxidative Stress: This condition occurs when there is an excess of reactive oxygen species (ROS) relative to the body's ability to counteract them.
ROS can cause harm to cellular structures, particularly photoreceptor cells.
Oxidative Stress in RP: It is thought that excessive oxidative stress plays a role in the progression of RP.
Antioxidant therapies have been investigated as possible treatments to alleviate oxidative damage.
Stem Cells: These are unspecialized cells that possess the potential to differentiate into various types of cells, including photoreceptor cells.
The application of stem cells in the context of retinitis pigmentosa (RP) is under investigation, with researchers aiming to replace damaged photoreceptor cells in affected patients.
This method may involve either the direct injection of stem cells into the ocular region or the activation of existing stem cells within the eye to transform into photoreceptor cells.
The Interconnection
These four components are intricately linked:
Fasting and Autophagy: The process of fasting can initiate autophagy, potentially reducing oxidative stress and supporting the longevity of photoreceptor cells.
Oxidative Stress and Stem Cells: Elevated oxidative stress levels can harm stem cells, diminishing their ability to develop into photoreceptor cells.
Stem Cells and Autophagy: Autophagy may be crucial in managing the survival and differentiation of stem cells.
In conclusion, fasting, autophagy, oxidative stress, and stem cells are all critical factors in the development of retinitis pigmentosa.
Gaining insights into these elements may facilitate the creation of new therapeutic strategies to slow or prevent the advancement of this disease
Dysregulated autophagy plays a significant role in a range of ocular disorders, such as retinitis pigmentosa (RP), cataracts, and glaucoma.
Additionally, oxidative stress, which arises from an imbalance between the production of reactive oxygen species (ROS) and the body's antioxidant defenses,
is a crucial element in retinal diseases.
This condition can worsen retinal injury and is closely associated with dysfunction in autophagy.
The relationship between oxidative stress and autophagy is vital in the development of ocular diseases, as oxidative stress can both trigger and be alleviated by autophagic processes
Fasting and Autophagy: The act of fasting is recognized for its ability to stimulate autophagy, which facilitates the elimination of damaged cells and diminishes oxidative stress.
may help counteract the effects of diseases like retinitis pigmentosa by supporting the longevity of retinal cells.
Stem Cells in Retinitis Pigmentosa
Role of Stem Cells: Stem cells represent a promising therapeutic strategy for retinitis pigmentosa.
They have the potential to regenerate damaged retinal cells, thereby restoring vision.
Challenges and Progress:
Although stem cell therapy shows great promise, it faces challenges, such as ensuring the survival and correct integration of transplanted cells into the retinal environment.
In summary
autophagy and oxidative stress are vital factors in the realm of retinal health and diseases like retinitis pigmentosa.
Fasting-induced autophagy may confer protective effects,
while stem cell therapy continues to be an encouraging area of investigation for restoring vision in individuals affected by RP
fasting day after other david fasting 4-3 days per week worth trial
fasting Autophagy stem cells and Oxidative Stress benefit
with or without other treatment to be evaluated within 6 month of fasting
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6070619/
https://link.springer.com/chapter/10.1007/978-1-4615-1897-6_1
https://www.nature.com/articles/4400210.pdf
https://www.sciencedirect.com/science/article/pii/S0098299721000984
https://cdnsciencepub.com/doi/10.1139/o94-066
https://emedicine.medscape.com/article/1227488-overview
fasting day after other, retinitis pigmentosa ,Autophagy ,stem cells , Oxidative Stress benefit ,Interconnection ,age of blindness ,treatment ,update ,inheritance ,diagnosis ,symptoms ,causes ,hereditary, no cure , complications ,you can't prevent RP,
Frequently Asked Questions (FAQs) About Retinitis Pigmentosa (RP)
What is Retinitis Pigmentosa (RP)?
Retinitis Pigmentosa (RP) is a group of inherited genetic disorders that affect the retina.
It results from mutations in approximately 100 different genes, leading to a progressive deterioration of photoreceptor cells (rods first, then cones).
This degeneration causes irreversible vision loss, often beginning in childhood and potentially resulting in legal blindness by the age of 40.
What are the primary causes of RP?
The main causes include inherited genetic mutations (autosomal dominant, recessive, or X-linked patterns) and drug-induced damage (e.g., antimalarials like hydroxychloroquine and ethambutol).
These drugs may mimic RP symptoms by harming the retinal pigment epithelium (RPE).
What are the early and advanced symptoms of RP?
Early Symptoms: Night blindness and loss of peripheral vision ("tunnel vision").
Advanced Symptoms: Blurred central vision, frequent tripping, macular edema, and cataracts.
How is RP diagnosed?
RP is diagnosed using clinical tools such as:
Fundoscopy: Reveals pigment deposits resembling "bone spicules."
Electroretinography (ERG): Measures reduced retinal electrical responses.
Genetic Testing: Identifies causative mutations.
Is there a cure for RP? What treatments are currently available?
There is no cure for RP, but current therapies aim to slow disease progression:
Vitamin A Palmitate: May delay degeneration in some patients.
Low-Vision Aids: Magnifiers, wearable tech, and mobility training.
Gene Therapy: FDA-approved Luxturna® for RPE65 mutations.
Retinal Implants: Devices like Argus II for advanced cases.
What emerging therapies are being researched for RP?
Autophagy Enhancement: Intermittent fasting to boost cellular "cleanup" processes.
Antioxidants: N-acetylcysteine (NAC), coenzyme Q10, and lutein to combat oxidative stress.
Stem Cell Therapy: Replacing lost photoreceptors with stem cell-derived cells.
What role do autophagy and oxidative stress play in RP? How can they be targeted for treatment?
Autophagy: A cellular "cleanup" process that removes damaged cells. RP involves autophagy dysfunction, worsening oxidative stress.
Oxidative Stress: Excess reactive oxygen species (ROS) accelerate photoreceptor death.
Targeted Therapies: Intermittent fasting to enhance autophagy and antioxidants to reduce ROS damage.
What are the common risks/complications of RP, and what practical advice is recommended for patients?
Complications: Early-onset cataracts, macular edema, and progressive vision field narrowing (<20° central field = legal blindness).
Advice:
Monitor experimental fasting protocols under medical supervision.
Consider combining autophagy boosters (e.g., fasting), antioxidants, and low-dose vitamin A.
Explore clinical trials for stem cell or gene therapies via platforms like ClinicalTrials.gov.
For personalized guidance, always consult an ophthalmologist or retinal specialist.
What is Retinitis Pigmentosa?
RP is a group of inherited retinal disorders caused by mutations in ~100 genes, leading to progressive degeneration of photoreceptor cells (rods first, then cones). This results in irreversible vision loss, often starting in childhood and potentially leading to legal blindness by age 40.
Key Characteristics
Causes:
Genetic Mutations: Autosomal dominant (most common), recessive, or X-linked inheritance.
Drug-Induced Damage: Antimalarials (e.g., hydroxychloroquine), ethambutol, and others may mimic RP by harming retinal pigment epithelium (RPE).
Symptoms:
Early: Night blindness, peripheral vision loss ("tunnel vision").
Advanced: Central vision blurring, frequent tripping, macular edema, and cataracts.
Diagnosis:
Clinical Tools: Fundoscopy (pigment deposits resembling "bone spicules"), electroretinography (reduced retinal response), genetic testing.
Current Management Strategies
No Cure Exists, but interventions aim to slow progression:
Vitamin A Palmitate: May delay degeneration in some patients.
Low-Vision Aids: Magnifiers, wearable tech, and mobility training.
Gene Therapy: FDA-approved Luxturna® for RPE65 mutations (restores partial vision).
Surgical Options: Retinal implants (e.g., Argus II) for advanced RP.
Emerging Therapeutic Frontiers
1. Autophagy and Fasting
Autophagy: A cellular "cleanup" process that removes damaged photoreceptors. RP is linked to autophagy dysfunction, exacerbating oxidative stress.
Fasting: Preclinical studies suggest intermittent fasting (3–4 days/week) may boost autophagy, reduce oxidative damage, and prolong photoreceptor survival.
Potential Protocol: "David fasting" (alternate-day or time-restricted feeding) under medical supervision.
2. Oxidative Stress
Role in RP: Excess reactive oxygen species (ROS) accelerate photoreceptor apoptosis.
Antioxidant Therapies: N-acetylcysteine (NAC), coenzyme Q10, and lutein are under study to neutralize ROS.
3. Stem Cell Therapy
Goal: Replace lost photoreceptors using stem cells (e.g., iPSCs, retinal progenitor cells).
Challenges: Ensuring cell integration, survival, and functional connectivity in the retina.
Progress: Early-phase trials show partial vision recovery in some patients.
Interconnected Mechanisms
Mechanism
Role in RP
Therapeutic Potential
Autophagy
Removes dysfunctional cells; impaired in RP.
Fasting, rapamycin analogs.
Oxidative Stress
Drives photoreceptor death via ROS accumulation.
Antioxidants (e.g., vitamin E, NAC).
Stem Cells
May regenerate retinal tissue; hindered by oxidative stress.
Cell transplantation, endogenous activation.
Complications & Prognosis
Common: Cataracts (early onset), macular edema, and progressive vision field constriction (<20° central field = legal blindness).
Variable Progression: Some retain central vision for decades; others lose functional sight by adulthood.
Inheritance Patterns
Autosomal Dominant: 50% risk to offspring if one parent carries the mutation.
X-Linked: Males more severely affected; females often carriers.
Practical Considerations for Patients
Fasting Trials: While promising in theory, human data are limited. Monitor effects over 6 months with a healthcare team.
Combination Therapies: Pair fasting/autophagy boosters with antioxidants and low-dose vitamin A.
Clinical Trials: Explore stem cell/gene therapy trials via platforms like ClinicalTrials.gov.
Conclusion
RP remains incurable, but emerging strategies—fasting-induced autophagy, stem cells, and antioxidant therapies—offer hope for slowing degeneration. Collaborative care involving geneticists, ophthalmologists, and nutritionists is critical. Always consult a specialist before adopting experimental regimens.
“The eye is the lamp of the body; so if the eye is healthy, the whole body will be full of light.” – Insights into RP research echo this timeless urgency to preserve vision.
Retinitis Pigmentosa (RP), is a hereditary retinal disorder , genetic mutations (affecting ~100 genes) , progressive degeneration of photoreceptor cells (rods and cones), resulting in symptoms , night blindness, tunnel vision, , eventual central vision loss, Key mechanisms , autophagy dysfunction ,oxidative stress, which accelerate retinal damage, Current management focuses , low-vision aids, vitamin A palmitate, and gene therapy (e.g., Luxturna®), while emerging strategies ,stem cell therapy, intermittent fasting (to boost autophagy), and antioxidants (e.g., NAC, lutein),Complications , legal blindness (central field <20°), cataracts, and macular edema are common, Research emphasizes, clinical trials , novel therapies , retinal implants , combinatorial approaches targeting autophagy, oxidative stress, and genetic repair,.