Brain Cell Damage : Fasting
Explore the latest treatment options for brain cell damage, including fasting, neuroplasticity, gene therapy, and stem cell therapy. Learn how these techniques can help repair damage and promote he...
NERVOUS SYSTEM
Dr Hassan Al Warraqi
10/2/2023
Latest Treatment Options for Brain Cell Damage: Fasting,
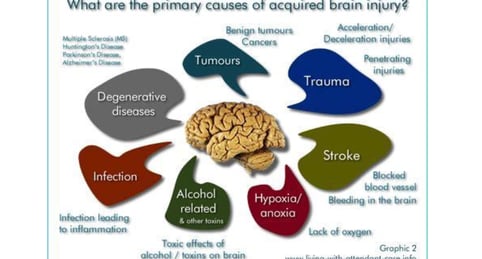
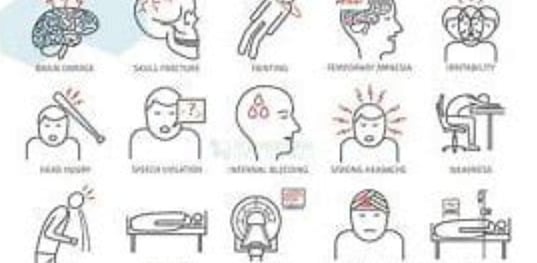
Brain cell damage is the destruction or deterioration of brain cells. It can occur due to a variety of factors, including:
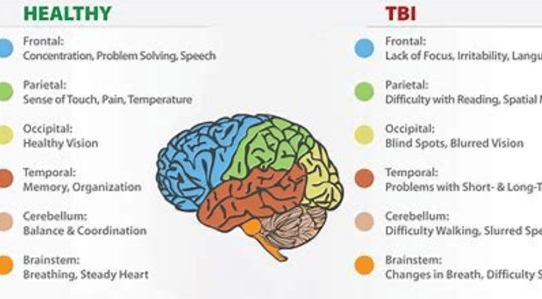
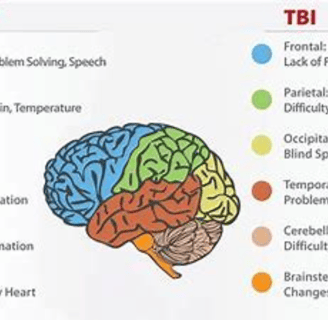
Traumatic brain injury (TBI): TBI is caused by a blow or jolt to the head. It can damage brain cells directly or by disrupting blood flow to the brain.
Stroke: A stroke occurs when the blood supply to part of the brain is blocked or interrupted. This can cause brain cells to die.
Infections: Some infections, such as meningitis and encephalitis, can damage brain cells.
Brain tumors: Brain tumors can grow and press on brain cells, damaging them.
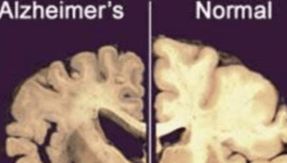
Neurodegenerative diseases: Neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease, are caused by the progressive loss of brain cells.
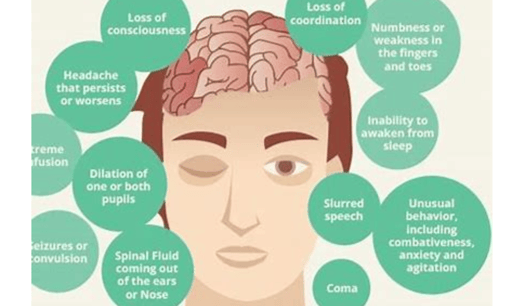
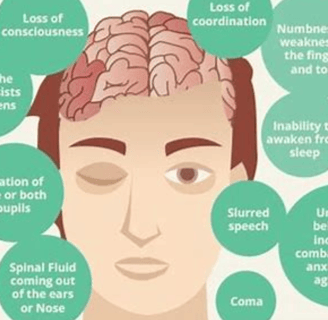
Brain cell damage can lead to a variety of symptoms
depending on the area of the brain that is affected. Some common symptoms include:
Memory loss
Difficulty concentrating
Problems with speech and language
Changes in mood or personality
Weakness or paralysis
Sensory problems
Seizures
brain fog
shallow memory
There is no cure for brain cell damage, but there are treatments available that can help to manage the symptoms and improve quality of life
Research on brain cell damage
Researchers are actively working to develop new treatments for brain cell damage some are:
Stem cell therapy: Stem cells have the potential to repair damaged brain cells. Researchers are working on ways to safely and effectively deliver stem cells to the brain to treat brain cell damage.
Gene therapy: Gene therapy could be used to deliver genes that protect brain cells from damage or promote their repair.
Neuroplasticity: Neuroplasticity is the brain's ability to change and adapt. Researchers are working on ways to harness neuroplasticity to help the brain compensate for brain cell damage.
Preventing brain cell damage
Avoid head injuries: Wear a helmet when participating in activities that could lead to a head injury.
Eat a healthy diet: A diet that is rich in fruits, vegetables, and whole grains can help to protect the brain from damage.
Exercise regularly: Exercise has been shown to improve brain health and reduce the risk of cognitive decline.
Manage stress: Stress can damage the brain. Managing stress through healthy coping mechanisms can help to protect the brain from damage.
If you are concerned about your risk of brain cell damage, they can help you to develop a plan to reduce your risk and protect your brain health.
Treatment for brain cell damage will vary depending on the underlying cause and the severity of the damage.
some common treatment options include:
Medication: Medications can be used to manage the symptoms of brain cell damage, such as pain, seizures, and mood swings.
Therapy: Therapy can help people with brain cell damage to learn coping skills and manage their symptoms. Therapy can also help people to regain lost skills and abilities.
Lifestyle changes: Lifestyle changes, such as eating a healthy diet, exercising regularly, and getting enough sleep, can help to improve brain health and reduce the risk of further damage. and stroke,
there are specific treatments
For some types of brain cell damage, such as TBrity of the damage and improve recovery. For example, surgery may be necessary to remove a blood clot or relieve pressure on the brain.
In addition to the above treatments, there are a number of emerging therapies that are being investigated for the treatment of brain cell damage. These therapies include:
Stem cell therapy: Stem cells have the potential to repair damaged brain cells. Researchers are working on ways to safely and effectively deliver stem cells to the brain to treat brain cell damage.
Gene therapy: Gene therapy could be used to deliver genes that protect brain cells from damage or promote their repair.
Neuroplasticity: Neuroplasticity is the brain's ability to change and adapt. Researchers are working on ways to harness neuroplasticity to help the brain compensate for brain cell damage.
If you have been diagnosed with brain cell damage. There may be new therapies available that can help you to improve your quality of life.
fasting
The only solution to repair the damage of autophagy and stem cells islamic one Neuroplasticity is the brain's ability for repair and heel .
intermittent fasting is the second choices.
fasting should be at least for 4 days per week or more
key words
brain cell damage,fasting,neuroplasticity,gene,stem,cells,autophagy, treatment options,stress,degeneration, repair the damage of autophagy and ,stem cells ,islamic one ,Neuroplasticity ,is the brain's ability for repair, heel,Gene therapy,Stem cell therapy,
Emerging Treatments for Traumatic Brain Injury and the Effects of Fasting
This document summarizes key insights from sources exploring emerging treatments for traumatic brain injury (TBI) and the role of intermittent fasting in brain health and neurological conditions.
Part 1: Emerging Treatments for Traumatic Brain Injury
This section examines pharmacological approaches under study for TBI, focusing on calcium channel blockers, excitatory amino acid (EAA) inhibitors, statins, and nitric oxide.
Calcium Channel Blockers:
Objective: Prevent cerebral vasospasm, maintain brain blood flow, and reduce further damage.
Nimodipine: Used since 1984 for vasospasm post-TBI. Trials (HIT 1, 2, 4) showed no clear benefits, with similar poor outcomes (39% vs. 40% placebo) and increased adverse reactions in traumatic subarachnoid hemorrhage cases.
Quote: "These results do not support a beneficial effect of nimodipine on outcomes in patients with traumatic subarachnoid hemorrhage."
SNX-111 (Ziconotide): Improved mitochondrial function in mice but was halted in trials due to higher mortality (25% vs. 15% placebo) from hypotension.
SNX-185: Direct hippocampal injection in rodents reduced neuronal damage and improved outcomes, but human application remains challenging.
Excitatory Amino Acid (EAA) Inhibitors:
Mechanism: TBI causes excessive glutamate release, leading to NMDA receptor overstimulation, ionic imbalance, and neuronal death.
Amantadine: Enhanced functional recovery in diffuse axonal injury patients, improving arousal and cognition at 200-400 mg/day.
Quote: "Future controlled studies will better define amantadine’s role in early arousal."
Dextromethorphan: Safe but ineffective in Phase III trials for TBI treatment.
Statins:
Mechanism: Enhance Akt, GSK-3β, CREB phosphorylation, increase BDNF and VEGF, and improve spatial learning.
Lovastatin: Reduced brain damage and inflammation in rats.
Atorvastatin/Simvastatin: Decreased neurological deficits and hippocampal damage while improving cerebral blood flow.
Nitric Oxide (NO):
Role: Modulates sensory-motor functions, cerebral blood flow, and neuroprotection.
Effects: nNOS-derived NO inhibits neurogenesis; eNOS/iNOS-derived NO promotes it. Statins like atorvastatin enhance eNOS expression.
Quote: "Further clarification is needed on the effects of nitric oxide synthase isoforms on neural plasticity."
Part 2: Fasting as a Treatment for Neurological Disorders
This section explores fasting types and their metabolic and neurological benefits.
Metabolic and Neurological Benefits:
Fasting improves glucose levels, insulin sensitivity, and neuronal resistance to toxins, surpassing caloric restriction benefits.
Human studies showed greater insulin sensitivity improvements in fasting individuals.
Quote: "Fasting may confer cellular metabolic benefits beyond caloric restriction."
Fasting Regimens:
Factors: Intensity, frequency, and duration of fasting.
Types: Pure fasting, intermittent fasting (4-12 hour eating windows), alternate-day fasting, two-day weekly fasting, periodic fasting, and voluntary fasting.
Water-Only/Liquid-Only Fasting: Allows water or calorie-free liquids, maintaining metabolic fasting states.
Misconceptions:
Short-term fasting reduces hunger and boosts energy, unlike severe caloric restriction, which causes fatigue.
Fasting increases basal metabolic rate by 5-15% initially, while chronic caloric restriction lowers it.
Prolonged fasting may induce temporary insulin resistance to preserve brain glucose, distinct from pathological resistance.
Part 3: Fasting and Anti-Inflammatory Effects
Study: Mice on low-fat (LFD) or high-fat diets (HFD) fasted for 24 hours.
Findings:
Fasting reduced body weight and glucose levels.
HFD increased leptin and body weight.
Fasting impaired spatial memory in HFD mice but not depression-like behavior.
In LFD mice, fasting boosted anti-inflammatory genes (IL-10) and reduced pro-inflammatory genes (IL-1β).
Quote: "Fasting impairs memory but does not affect depression-like behavior in mice fed a high-fat diet."
Part 4: Benefits of Intermittent Fasting for the Brain
Definition: Eating pattern with no-calorie periods.
Focus: Time restriction, allowing any healthy diet during eating windows.
Part 5: Intermittent Fasting and Hippocampal Neurogenesis
Study: Intermittent fasting (12-16 hour or alternate-day fasting) in mice for 3 months increased hippocampal neurogenesis markers (NeuN, Nestin, PSD95).
Quote: "Intermittent fasting increases neurogenesis in the adult hippocampus."
Part 6: Intermittent Fasting and Stroke Neuroprotection
Mechanisms: Enhances glucose tolerance, blood pressure control, and gene expression for plasticity and neuroprotection while reducing inflammation.
Quote: "Intermittent fasting can enhance stroke tolerance by reducing oxidative stress and inflammation."
Part 7: Neural Plasticity
Definition: Brain’s ability to adapt structure and function to experience or injury.
Types: Structural (synaptic changes) and functional (sensory/motor map reorganization).
Applications: Brain damage recovery, sensory adaptation, and chronic pain management.
Part 8: BDNF and Insulin Resistance in Vitiligo
Study: Vitiligo patients had higher BDNF levels, correlating with disease duration, BMI, glucose, and insulin resistance.
Quote: "The vitiligo group showed significantly higher blood BDNF levels."
Part 9: Fasting Types, Risks, and Benefits
Types:
Intermittent fasting (TRF, ADF, MADF, periodic).
Prolonged fasting (>2 days).
Voluntary fasting.
Risks: Unsafe for those with low blood pressure or eating disorders.
Benefits: Weight management, reduced inflammation, improved insulin sensitivity.
Quote: "Intermittent fasting is common for weight management and reducing inflammation."
Part 10: Key Points
Intermittent fasting may promote neuronal regeneration and BDNF production.
It reduces brain inflammation and enhances cognition.
Fasting is a cultural/religious practice with health benefits like ketosis and stress resistance.
Early research suggests medical applications, but more studies are needed.
Conclusions
Pharmacological TBI treatments show mixed results, requiring further research.
Intermittent fasting holds promise for brain health, but human studies are needed to confirm mechanisms and applications.
Consult healthcare professionals before starting fasting, especially with underlying conditions.
Q&A on Treatments for Traumatic Brain Injury and the Neurological Effects of Fasting FAQS
Traumatic Brain Injury (TBI)
Q: What is the role of calcium channel blockers in treating traumatic brain injury?
A: Calcium channel blockers aim to prevent cerebral vasospasm post-injury, maintain brain blood flow, and reduce further damage.
For example, nimodipine has been studied since 1984 for this purpose. However, clinical trial results are mixed.
Some analyses suggested potential benefits in patients with traumatic subarachnoid hemorrhage, but others found no consistent benefits and noted increased adverse reactions. Research on N-type calcium channel blockers like ziconotide (SNX-111) and SNX-185 shows preclinical promise in improving mitochondrial function and reducing neuronal damage, but human application faces challenges due to side effects and administration methods.
A: TBI reduces cerebral blood flow, depleting energy stores and disrupting membrane polarization.
This triggers excessive release of excitatory amino acids, particularly glutamate, which overstimulates NMDA receptors.
This leads to ionic imbalance, excessive calcium influx into neurons, and cell death. EAA inhibitors aim to interrupt this cascade before neuronal damage becomes irreversible.
Drugs like amantadine and dextromethorphan (HU 211) have been studied. Amantadine appears to safely improve arousal and cognition, while dextromethorphan showed no efficacy in clinical trials despite being safe.
Q: What is the potential role of statins in treating traumatic brain injury?
A: Studies suggest statins (e.g., simvastatin, lovastatin, atorvastatin) may have protective effects post-TBI.
These include increasing phosphorylation of proteins involved in cell survival and growth,
boosting expression of neurotrophic factors like BDNF and VEGF, promoting cell proliferation and differentiation in the hippocampus, and improving recovery from spatial learning deficits.
Statins may also reduce inflammation and enhance cerebral blood flow after injury.
Q: How can nitric oxide (NO) affect the brain after a traumatic brain injury, and can this pathway be targeted therapeutically?
A: Nitric oxide is a critical molecule in the central nervous system, involved in regulating cerebral blood flow, neuroprotection, and neurotoxicity. Its role in neurogenesis post-TBI is complex and depends on the nitric oxide synthase (NOS) enzyme producing it.
For instance, nNOS-derived NO inhibits neurogenesis, while eNOS- and iNOS-derived NO may promote it.
Treatments like atorvastatin can increase eNOS production, elevating cGMP levels linked to neuronal proliferation.
Further research is needed to clarify the distinct effects of NOS isoforms on neural plasticity, cell survival, and neurological functions post-TBI.
Fasting and Its Neurological Effects
Q: What are the different types of fasting studied and their potential effects on brain health?
A: Fasting types include intermittent fasting (IF) and prolonged fasting.
Intermittent fasting encompasses time-restricted feeding (TRF), alternate-day fasting (ADF), modified alternate-day fasting (MADF), and periodic fasting.
Studies show fasting, particularly IF, can improve insulin sensitivity, enhance neuronal stress resistance, stimulate hippocampal neurogenesis, and offer neuroprotection against acute brain injuries like stroke and neurodegenerative diseases.
Q: How does fasting differ from severe caloric restriction in terms of metabolic and sensory effects?
A: Fasting differs significantly from severe caloric restriction.
Short-term fasting often leads to reduced hunger, improved energy, mood, self-confidence, and quality of life.
In contrast, severe caloric restriction is associated with persistent hunger, fatigue, irritability, apathy, and reduced libido. Metabolically, chronic caloric restriction lowers basal metabolic rate, while fasting initially increases it slightly.
Q: What is "fasting-induced insulin resistance," and what is its physiological significance?
A: Fasting-induced insulin resistance, also called "starvation diabetes," is a temporary reduction in insulin sensitivity in skeletal muscle, typically after fasting beyond 48 hours.
It occurs with low blood sugar and insulin levels, limiting muscle glucose uptake to ensure a steady glucose supply for the brain’s essential needs.
This is a normal physiological adaptation to preserve brain function, distinct from pathological insulin resistance.
Q: How can intermittent fasting contribute to neuroprotection after stroke and enhance neural plasticity?
A: Intermittent fasting may offer neuroprotection post-stroke through multiple mechanisms.
It stimulates beneficial genes that promote neural plasticity, regeneration, and protection while reducing inflammation, oxidative stress, and apoptosis.
It also enhances neural plasticity, improving long-term functional outcomes.
These benefits may stem from increased production of neurotrophic factors like BDNF and VEGF and activation of the Notch pathway, which supports neurogenesis.